Powered by purpose
A platform for immune-fibrosis innovation
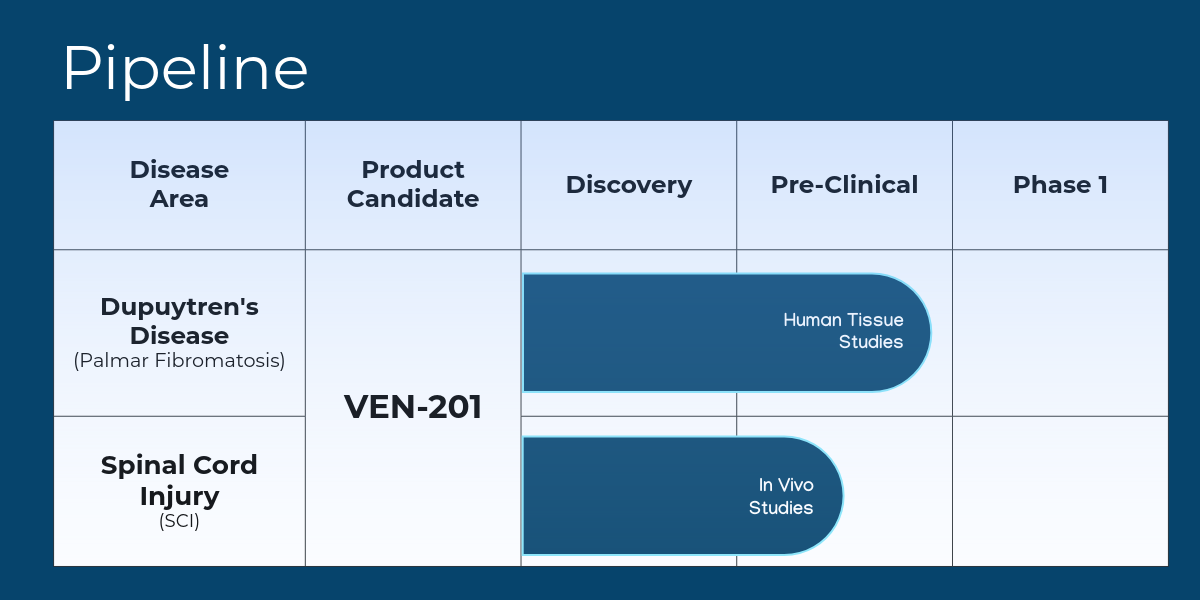
Ventoux Biosciences is advancing VEN‑201, a novel, locally delivered therapy designed to disrupt immune‑fibrotic pathways and restore tissue function. Our patented platform begins with Dupuytren’s disease, where VEN‑201 is positioned as a first‑line treatment for early‑stage disease and recurrence prevention. Building on this foundation, we are expanding into central nervous system fibrosis, with a strategic focus on spinal cord injury. With 18–24 months to first‑in‑human studies, our pipeline reflects both immediate patient need and long‑term vision.
Pipeline
VEN‑201, an investigational compound, has demonstrated key anti‑fibrotic activity and a favorable safety profile in a pre‑clinical in vivo dermal fibrosis (scleroderma) model. Building on these results, we are advancing VEN‑201 as our patented lead developmental candidate for Dupuytren’s disease, where localized, disease‑modifying therapy has the potential to reduce surgical burden and restore tissue function.
The investigational products listed on this page are not approved by the FDA or have not been approved for the above referenced indication, and the safety and effectiveness of such has not been established.
VEN‑201 was identified through targeted scientific evaluation, expert insight, and artificial intelligence data analysis. Our goal was to uncover previously approved treatments with new potential as anti‑fibrotic therapies for Dupuytren’s disease and related under‑served connective tissue conditions. The reference compound for VEN‑201 has been approved by the FDA and EMA for other indications for more than 20 years, establishing a proven safety profile. Based on pre‑clinical evaluation, we are advancing VEN‑201 as a patented, repurposed, and reformulated disease‑modifying agent with a novel route of administration — positioned to move forward via an expedited regulatory pathway. In parallel, we are exploring VEN‑201’s potential to modulate fibrotic barriers in spinal cord injury, where fibrosis impedes neural regeneration and limits recovery.





